Scaffold-Free soft Tissue Engineering
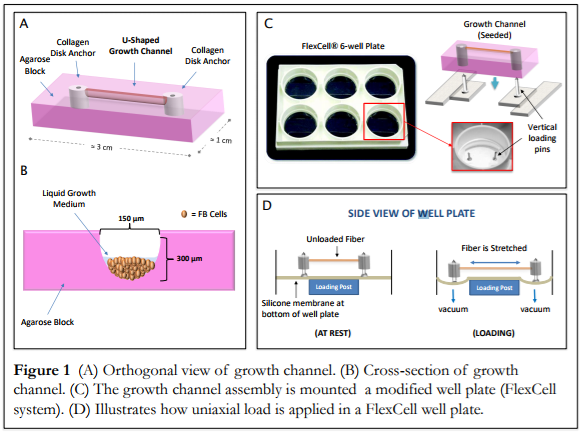
Part of the Corr Group's research is the development of scaffold-free soft tissue engineering models. This embryonic-inspired approach to tissue generation utilizes micromolded, differentially-adherent growth channels to guide seeded cells into single, naturally-formed fibers. We have also developed a custom bioreactor to mechanically and electrically stimulate our fibers. The application of these stimuli during fiber development enables precise control over the structural and/or mechanical properties of the mature fiber.
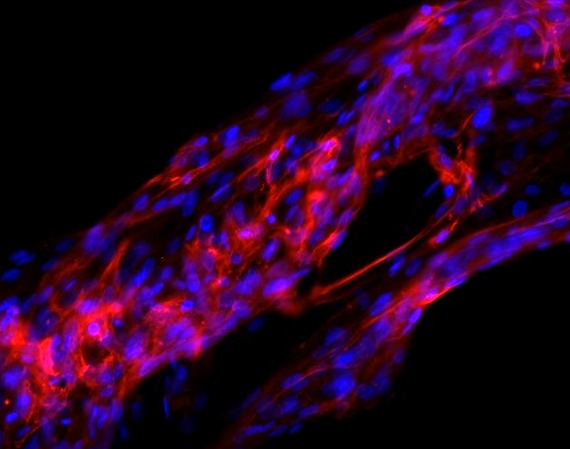
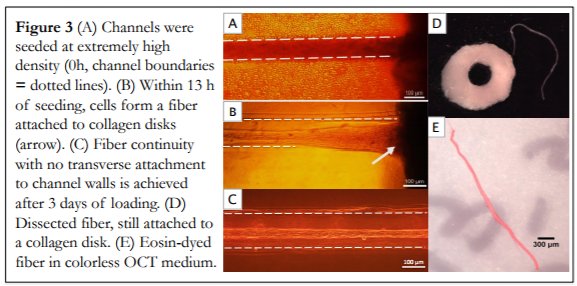
The scaffold-free, embryonic-inspired technique we are exploring relies on a highly cellular microenvironment with little matrix presence to encourage direct cell-cell contact an native matrix production. Our approach has been successfully applied to synthesize long-lasting (> 21 day survival) individual tendon fibers with good mechanical properties. We note that key to single tendon fiber synthesis is early mechanical stimulation, e.g., intermittent cyclic uniaxial strain.
Changes in tendon fiber alignment and nuclear morphology in response to uni-axial loading.
We have also been able to create single muscle fibers capable of force production using this method. Similar to the tendons, they had more favorable mechanical properties with mechanical and electrical stimulation.
Current projects include evaluation of mechanical and electrical stimuli on both engineered tendon and muscle fibers, namely characterization of the effects of these stimuli on parameters such as collagen content and cell nuclear morphology. We are also exploring the effect of crowding on these fibers.
Laser Direct-Write bioprinting
We have developed a laser-based direct-write (LDW) bioprinting technique capable of patterning living cells, in a spatially precise manner, while maintaining cellular viability. These patterns can consist of separate spherical microbeads or a microbead mat consisting of many beads that have been printed in an overlapping fashion to form one continuous shelled construct. Precise cell placement, especially of multiple cell types in co- or multi-cultures and in three dimensions, can enable research possibilities otherwise impossible, such as the cell-by-cell assembly of complex cellular constructs.
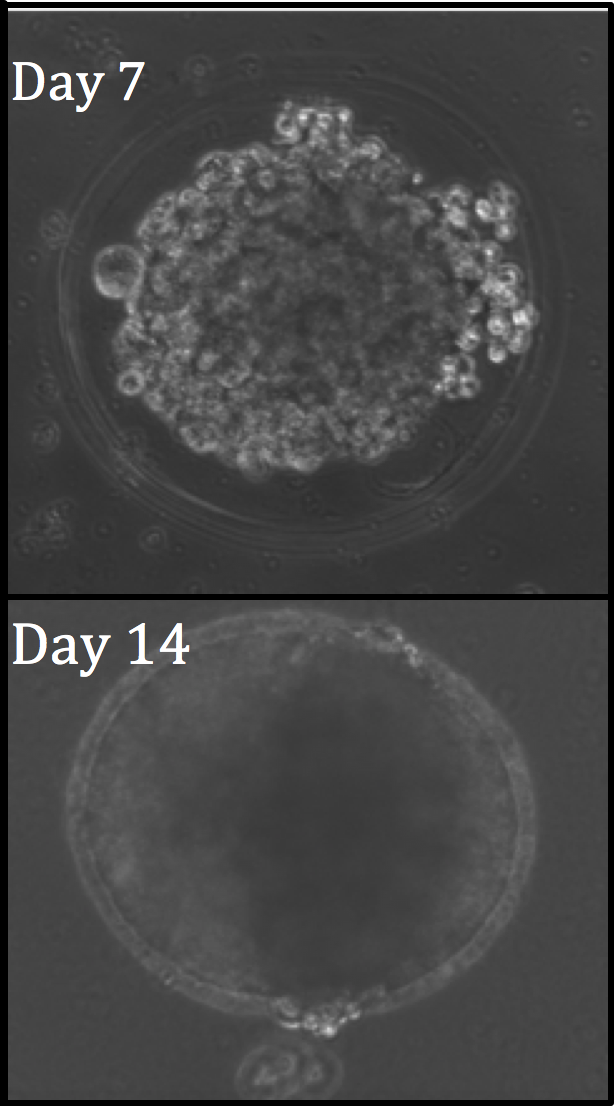
Our work with this technique has thus far enabled the fabrication of 3D customizable core-shelled environments, which allow for controlled cell-cell contact, cellular proliferation and aggregation within the capsule. In this way, we are exploring the properties of development of cellular aggregates, formed naturally within the shelled environments by self assembling cell types.
Current projects are leveraging these microbead mats to create constructs with spatially prescribed biomechanical and cellular heterogeneity.

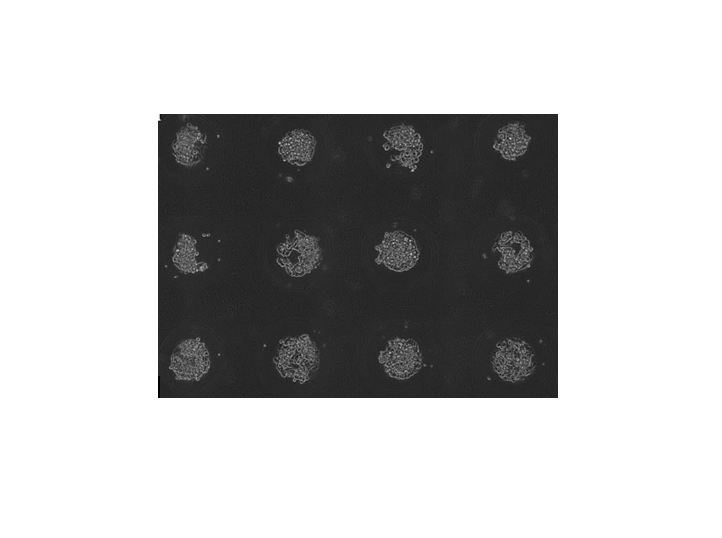
Optical Coherence Tomography/Elastography
Optical coherence tomography (OCT) allows for nondestructive, real time, 3D imaging of our tumor spheroids, which we use to evaluate sphericity and cell count over time. By applying cyclic micro-perturbations to the sample, this system can also be adapted for optical coherence elastography (OCE). This allows for measurment of biomechanical properties such as elastic modulus on a voxel level across the whole printed construct.